Develop safer drugs without unexpected toxicity
Frontier AI systems to predict, explain, and fix human toxicity risks better than animal testing.
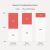
Toxicity is a leading cause of clinical trial failures
Every year, unexpected toxicity denies patients many effective medicines and costs industry billions of dollars.
Old Paradigm
40% - 50% of drugs fail because of toxicity.
-
90% of drugs fail clinical trials; clinical toxicity is a leading cause despite preclinical lab and animal toxicity experiments 1 SOURCE Impact of a five-dimensional framework on R&D productivity at AstraZeneca Nature Reviews Drug Discovery 2 SOURCE An analysis of the attrition of drug candidates from four major pharmaceutical companies Nature Reviews Drug Discovery 3 SOURCE Costs of Drug Development and Research and Development Intensity in the US, 2000-2018 JAMA Network Ope
-
Tens of billions of dollars are lost to late stage drug toxicity
Old Paradigm
Existing physical experiments are inaccurate and expensive.
-
Animal testing has poor predictive performance for human liver injury 4 SOURCE Current nonclinical testing paradigm enables safe entry to First-In-Human clinical trials: The IQ consortium nonclinical to clinical translational database Toxicology and Applied Pharmacology
-
Lab experiments show good specificity but lack sensitivity 5 SOURCE Setting Clinical Exposure Levels of Concern for Drug-Induced Liver Injury (DILI) Using Mechanistic in vitro Assays Toxicological Sciences 6 SOURCE Utility of spherical human liver microtissues for prediction of clinical drug-induced liver injury Archives of Toxicology
-
Experiments costs thousands to millions of dollars and take weeks to months to run

Changing Industry
Current experimental methods are becoming obsolete.
-
Congress dropped the legal requirement for animal testing7 SOURCE “The FDA no longer requires all drugs to be tested on animals before human trials” Science | AAAS
-
Congress and the FDA have announced they are phasing out animal experiments8 SOURCE "FDA Announces Plan to Phase Out Animal Testing Requirement" FDA Commissioner
-
Industry, big pharma, regulators, and academia are looking for alternative approaches

New Paradigm
It's time to accurately predict toxicity in humans.
-
AI that accurately assesses risk of a drug's toxicity in humans from the earliest stages of drug discovery and development
-
AI that explains reasons and mechanisms behind toxicity risk and drug exposure rather than just raising an alert